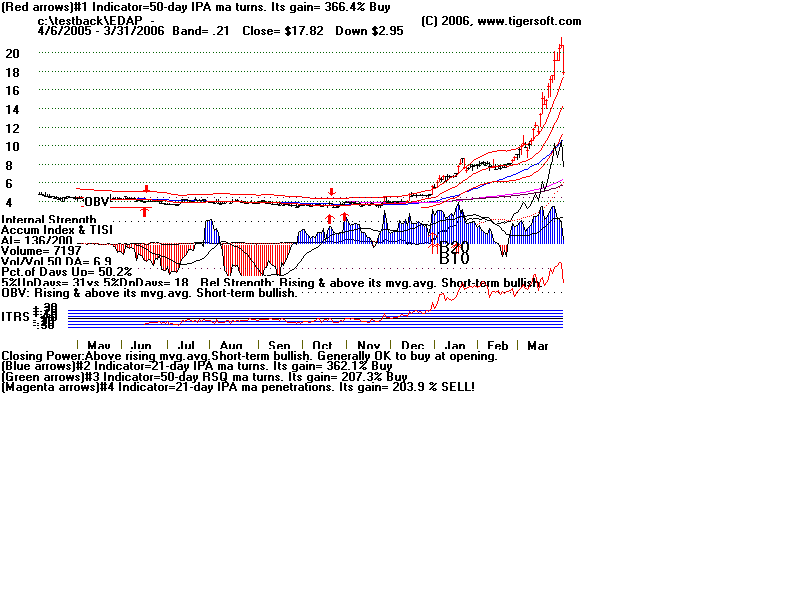
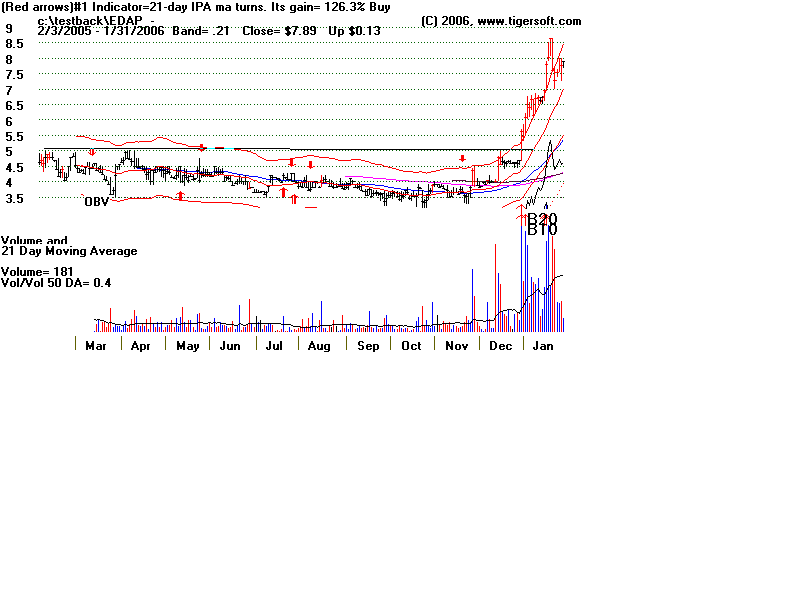
# EDAP TMS S.A. Reports Clinical Results
# 2005 Oct 31 | 7:52 PM
# EDAP TMS S.A. announced the advanced clinical data on the success of high intensity
focused ultrasound (HIFU) with Ablatherm. The study of more than 1,000 patients treated
with Ablatherm, showed a success rate up to 93.7% based on negative biopsies and a Nadir
PSA down to 0 for low and intermediate risk patients. These results were achieved just two
months post treatment, a significant improvement in the time to confirmable results as
compared to other therapies. Additionally, the results indicate that 70% of the patients
who opted for partial ablation of the prostate remained potent, once again well above the
normal ranges of other therapies
# EDAP TMS S.A. Signs HIFU with Ablatherm Contract with Major Hospital Group in France
Covering Six Clinics
# 2005 Feb 18 | 4:15 PM
# EDAP TMS S.A. announced the purchase of the first Ablatherm device by "La Mutualite
Francaise" which is responsible for more than 2,000 public clinics in France.
Starting with six sites throughout France, Ablatherm will be shared on a mobile basis,
making the HIFU treatment available in France to a broader population of patients
suffering from localized prostate cancer.
# EDAP TMS S.A. Cancer Treatment Technology To Be Made Available In North America
# 2005 Apr 11 | 4:00 AM
# EDAP TMS S.A. announced that it has entered into an exclusive sales agreement with
Canadian company, Maple Leaf HIFU, allowing them to market Ablatherm, the Company's
non-invasive treatment for prostate cancer, in Canada and develop access to Ablatherm-HIFU
technology throughout the Canadian territory. The non-invasive treatment will be available
to North American patients through a clinical installation in Toronto.